DE, UNITED STATES, February 17, 2026 /EINPresswire.com/ —
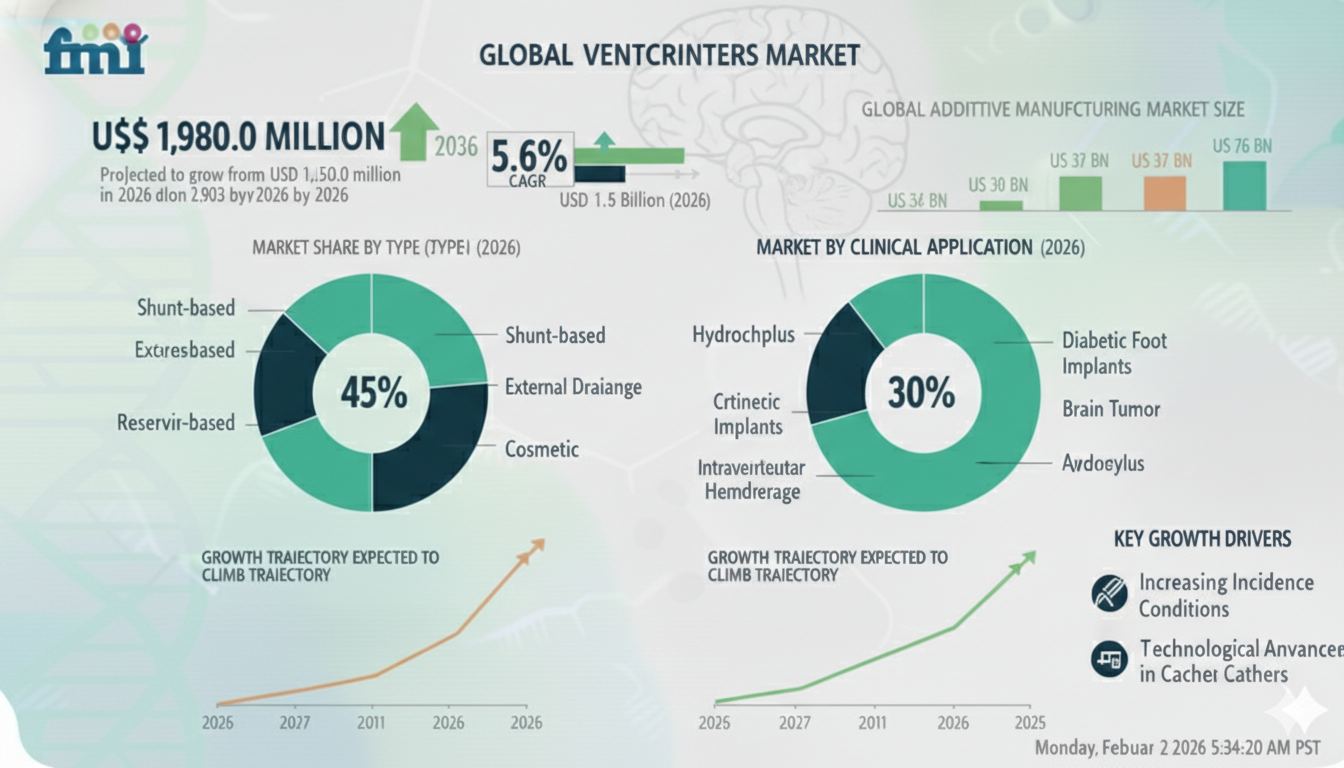
The global Ventricular Catheters Market is projected to grow from USD 1,150.0 million in 2026 to USD 1,980.0 million by 2036, reflecting a CAGR of 5.6% over the forecast period. Growth is being driven by sustained hospital-based neurocritical care demand for cerebrospinal fluid (CSF) drainage and intracranial pressure (ICP) management. As infection prevention and standardized ICU protocols gain prominence, the market is evolving toward coated and performance-consistent catheter technologies.
Why the Market is Growing
Demand for ventricular catheters is expanding as neurocritical care volumes remain elevated across tertiary and trauma-equipped hospitals. Hydrocephalus continues to dominate clinical utilization, supported by both pediatric and adult patient populations requiring long-term CSF diversion.
Infection mitigation strategies are reshaping procurement behavior. Antibiotic-impregnated and antimicrobial-coated catheters are increasingly prioritized, particularly in neuro-ICU environments where ventriculitis risk is closely monitored. Standardization of ICU protocols and performance-driven catheter selection are reinforcing consistent demand patterns.
Additionally, centralization of high-acuity neurological care into specialized facilities is reinforcing hospital-led demand concentration, ensuring recurring utilization of EVD and VP shunt catheter systems.
Segment Spotlight
Product Type: EVD Catheters Lead (~42.0%)
External Ventricular Drain (EVD) catheters account for approximately 42.0% of total market demand, making them the leading product category in the Ventricular Catheters Market. These devices are critical in emergency intracranial pressure management and acute cerebrospinal fluid diversion. Their role in neuro-ICU workflows, especially for trauma and hemorrhagic cases, secures their anchor position in hospital procurement strategies.
Indication: Hydrocephalus Dominates (46.0%)
Hydrocephalus represents 46.0% of total ventricular catheter utilization, reflecting its chronic nature and recurring treatment requirements. Long-term shunting procedures and lifetime management pathways make this indication the structural backbone of market demand. Infection prevention, catheter patency, and reliability over extended use remain core selection criteria.
End User: Hospitals at the Core
Hospitals remain the dominant end-user segment, particularly those equipped with neurosurgical and neuro-ICU capabilities. While Ambulatory Surgical Centers (ASCs) and specialty neuro centers contribute to elective and follow-up pathways, high-acuity neurocritical cases continue to concentrate demand within hospital infrastructures.
Drivers, Opportunities, Trends, Challenges
Drivers:
Sustained neurosurgical demand for CSF diversion in hydrocephalus, TBI, and hemorrhagic stroke is driving recurring catheter utilization. Hospital-based procedural concentration reinforces consistent product demand.
Opportunities:
Coating innovation—antibiotic-impregnated, antimicrobial (silver), and heparin/anti-thrombogenic variants—creates opportunities for manufacturers to differentiate on infection-risk mitigation and material reliability.
Trends:
Standardization of ICU workflows and infection-control protocols is accelerating adoption of coated catheters. Procurement decisions are increasingly aligned with institutional quality metrics and infection surveillance benchmarks.
Challenges:
Market differentiation is less about disruptive innovation and more about performance consistency, material integrity, and drainage reliability. Manufacturers must convert coating advancements into defensible clinical outcomes.
Competitive Landscape
The Ventricular Catheters Market is characterized by coating-based differentiation, infection-control alignment, and clinical reliability positioning. Leading players such as Medtronic, Integra LifeSciences, Johnson & Johnson (Codman Neuro), B. Braun, Sophysa, Natus Medical, Spiegelberg, Neuromedex GmbH, RAUMEDIC AG, Vygon, Teleflex, and Mizuho Medical are strengthening portfolios across EVD and VP shunt systems.
Recent developments include FDA approvals for coated EVD technologies and investments in navigation systems designed to improve catheter placement accuracy. Competitive positioning increasingly revolves around antimicrobial integration, thrombogenicity reduction, and ICU-centered usability enhancements.
Get Access of Report Sample:https://www.futuremarketinsights.com/reports/sample/rep-gb-32044
Scope of the Report
Market Size (2026): USD 1,150.0 million
Forecast Period: 2026–2036
Product Types: EVD Catheters, VP Shunt Catheters, ICP Monitoring Integrated Catheters, Specialty Ventricular Access Catheters
Coating Types: Standard, Antibiotic-impregnated, Antimicrobial (silver), Heparin / anti-thrombogenic
Indications: Hydrocephalus, Traumatic brain injury, ICH / IVH, SAH, CNS infection / other
End Users: Hospitals, Ambulatory Surgical Centers, Specialty Neurology Centers
Regions Covered: North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Countries Covered: USA, Germany, UK, France, Japan, China, India, and 40+ countries
Key Companies Profiled: Medtronic, Integra LifeSciences, Johnson & Johnson (Codman Neuro), B. Braun, Sophysa, Natus Medical
FAQ
What is the projected growth rate of the Ventricular Catheters Market?
The market is expected to expand at a CAGR of 5.6% between 2026 and 2036.
How large will the market be by 2036?
It is projected to reach USD 1,980.0 million.
Which product type leads the market?
EVD ventricular catheters lead with approximately 42.0% share.
What is the leading clinical indication?
Hydrocephalus dominates, contributing 46.0% of total usage.
Which country holds the largest share?
The United States holds the largest market share.
What is driving infection-control focused adoption?
Increased emphasis on ventriculitis risk reduction, coated catheter technologies, and standardized ICU protocols are shaping procurement decisions.
Explore More Related Studies Published by FMI Research:
Ventricular Fibrillation Treatment Market:https://www.futuremarketinsights.com/reports/ventricular-fibrillation-treatment-market
Airway Catheters Market:https://www.futuremarketinsights.com/reports/airway-catheters-market
Rectal Catheters Market:https://www.futuremarketinsights.com/reports/rectal-catheters-market
Balloon Catheters for Bile Stone Removal Market:https://www.futuremarketinsights.com/reports/balloon-catheters-for-bile-stone-removal-market
Centesis Catheters Market:https://www.futuremarketinsights.com/reports/centesis-catheters-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us – sales@futuremarketinsights.com
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()